Abstract
Introduction: The safety and efficacy of venetoclax (VEN) in combination with hypomethylating agents (HMA) in older adults with newly diagnosed acute myeloid leukemia (AML) or those ineligible for intensive chemotherapy is well established. Recently, VEN/HMA has emerged as a potential option in relapsed or refractory (r/r) AML. Although encouraging, data on whether this combination compares favorably to standard chemotherapy (SC) in r/r AML are limited.
Methods: This was a single-center, retrospective analysis from November 2012 to March 2022 that compared VEN/HMA to SC in r/r AML patients at a 923-bed tertiary care hospital with an outpatient cancer facility. The primary endpoint was overall response rate (ORR) defined as rate of complete remission (CR), complete remission with incomplete hematologic recovery (CRi), morphological leukemia-free state (MLFS), and partial remission (PR). Key secondary outcomes included overall survival (OS), relapse-free survival (RFS), and toxicity. Continuous variables were compared using the Mann-Whitney U test or the two-sample t-test and categorical variables were compared using the Chi-squared or Fisher's exact test. Time to event data were assessed using the Kaplan-Meier method. Log-rank test was used to compare the survival endpoints between groups. A p-value < 0.05 was considered statistically significant.
Results: A total of 281 patients underwent screening, of those, 63 adult patients were included in the analysis (VEN/HMA, n = 25 and SC, n = 38). The primary reason for exclusion was previously untreated AML or myelodysplastic syndrome. Baseline characteristics were balanced between the groups. Overall, 53 patients had de novo AML, 25 patients had received 3 or more prior lines of chemotherapy, 20 patients were ≥ 60 years, and 8 patients had undergone previous stem cell transplantation. In the VEN/HMA cohort, 32% had prior exposure to a hypomethylating agent and 36% received concomitant azole antifungals. The majority of patients in both groups were stratified as intermediate or poor risk according to the European LeukemiaNet (ELN) 2017 criteria. Common chemotherapy regimens included MEC (74%), ME (12%), and FLAG ± IDA (11%). Most patients in the VEN/HMA group received decitabine and of those, 60% initiated therapy in the outpatient setting.
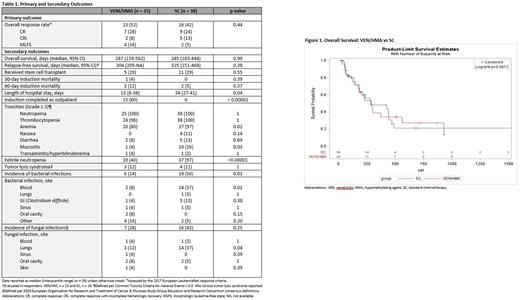
Treatment outcomes are shown in Table 1 and OS results are presented in Figure 1. Both therapies resulted in similar ORRs (VEN/HMA 52% vs SC 42%, p=0.44). Combined CR/CRi was achieved in 36% of patients in the VEN/HMA group and 37% of patients in the SC arm (p=0.95). Median time to response was 73 days for VEN/HMA and 45 days for SC (p=0.38). The OS between VEN/HMA and SC groups was not statistically different (p=0.99, log-rank test) with median OS of 287 days and 285 days, respectively. No difference in RFS was observed between the two groups (0.38, long-rank test) with median RFS of 304 days in the VEN/HMA arm and 325 days in SC group. Prior HMA exposure in VEN-treated patients was associated with a lower CR/CRi rate (13% vs 41%, p=0.09). Induction mortality rates at 30 and 60 days were similar between the groups. Duration of hospitalization was significantly shorter in patients who received VEN/HMA as compared to those who received SC (p=0.04).
In terms of safety, patients treated with VEN/HMA had lower rates of any grade diarrhea (40% vs 71%, p=0.01), any grade mucositis (20% vs 61%, p=0.001), and grade ≥ 3 anemia (80% vs 97%, p=0.02). The rate of febrile neutropenia was significantly higher in the SC group than in the VEN/HMA group (97% vs 40%, p<0.00001), as was the rate of bacterial infections (50% vs 24%, p=0.03). Fungal pneumonias were also reported more frequently in patients receiving SC (37% vs 12%, p=0.04). Lastly, a trend toward a longer median time to platelet recovery was noted in VEN/HMA patients receiving concomitant azole antifungals (38 days vs 26 days, p=0.52).
Conclusion: Treatment with VEN/HMA yielded similar outcomes compared with SC and was associated with lower infection rates, a potential advantage in a patient population at high risk for infectious complications. Additionally, safe initiation of this regimen in the outpatient setting and shorter duration of hospitalization when compared to SC could lead to a reduction in use of healthcare resources and improvement in quality of life. These findings add to a growing body of evidence that suggest VEN/HMA is non-inferior to SC with a favorable toxicity profile.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal